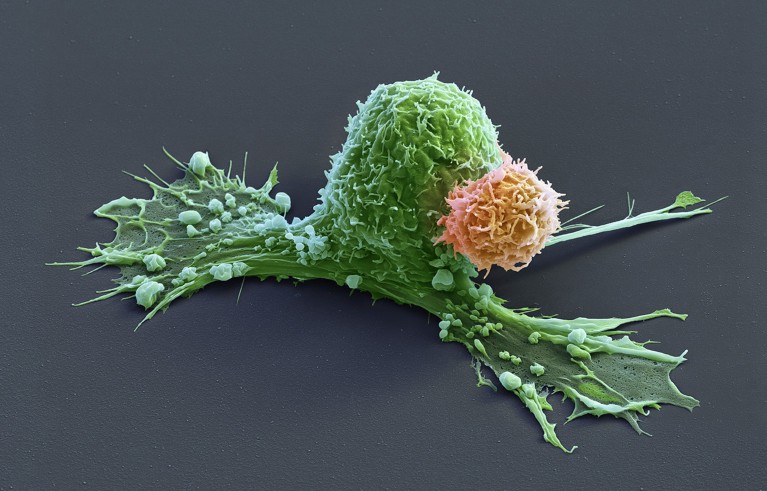
A CAR-T cell (orange) has attacks a cancer cell (green), which is starting to contract.Credit: Eye Of Science/Science Photo Library
The girl was four years old when she arrived at Texas Children’s Hospital in Houston to receive a highly experimental therapy for nerve-cell cancer. Standard treatments had been unable to hold the cancer back. It had spread to her bones, and the prognosis was poor.
The race to supercharge cancer-fighting T cells
Nineteen years later, she is cancer-free and the mother of two children. The remarkable success story, published on 17 February in Nature Medicine1, is the longest reported cancer remission following treatment with engineered immune cells called CAR T cells.
In the years since the girl’s treatment in 2006, CAR T cells have produced stunning results in some blood cancers such as leukaemia. Seven CAR-T-cell therapies have been approved by the US Food and Drug Administration since 2017, and some early recipients of CAR-T therapy have been cancer-free for more than a decade.
But researchers have struggled to repeat that success against solid tumours such as those caused by neuroblastoma, a nerve-cell cancer that is typically diagnosed in young children. That makes the latest results particularly good news, says Sneha Ramakrishna, a paediatric oncologist and cancer researcher at Stanford University School of Medicine in California, who was not involved in the study.
“This provides me with a lot of hope,” she says. “We’re going to unlock CAR T cells for people with solid tumours.”
Cell target
CAR T cells are immune cells that have been engineered to make a protein called a chimeric antigen receptor (CAR). This protein is designed to latch onto a target found on a cancer cell, triggering the immune cell to attack and destroy it.
Why are so many young people getting cancer? What the data say
When the Texas neuroblastoma study began in 2004, CAR-T-cell therapy was still a bit of a wild idea, says Helen Heslop, an immunotherapy researcher at Baylor College of Medicine in Houston, and a member of the team that ran the trial. “This is some kind of weird synthetic biology,” she remembers thinking. “Will it actually work?”
The CAR-T-cell therapies Heslop and her colleagues tested then are now considered first-generation editions of the molecules. Later versions, including those that are now approved medicines, contain extra modifications to bolster their power. Heslop calls her first CAR-T-cell study “a vintage trial”.
You Might Also Like
what we do and don’t know
We are 23 of the 27 original members of the Scientific Advisory Group for the Origins of Novel Pathogens (SAGO)...
Five ways increased militarization could change scientific careers
Ukrainian soldiers test drones in Donetsk, Febuary 2025.Credit: Serhii Mykhalchuk/Global Images Ukraine via GettyMilitary budgets are growing, especially in larger...
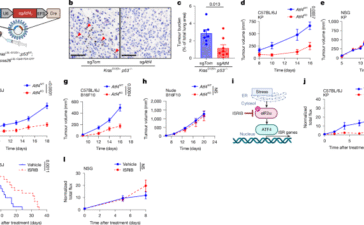
The integrated stress response promotes immune evasion through lipocalin 2
Cell linesThe KP cells used here were established previously66. Atf4 and Lcn2 knockout cell lines were generated by transient transfection...
How AI slop is causing a crisis in computer science
AI slop is flooding computer science journals and conferences.Credit: Quality Stock/AlamyFifty-four seconds. That’s how long it took Raphael Wimmer to...